

Thomsons experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thompson conducted his first cathode ray tube experiment to prove that rays emitted from an electron gun are inseparable from the latent charge. Light is created when electrons hit a fluorescent tube. In 18977 JJ Thomson performed several experiments to examine the structure of atom using the cathode ray application. The cathode ray tube (CRT) was invented in 1897 by the German physicist Karl Ferdinand Braun, is an vacuum glass envelope containing an electron gun, a source of electrons and a fluorescent light, generally with internal or external means in order to accelerate and redirect the electrons. How did it work and why did Thomson do the experiment in the first place. During cathode ray tube experiment a negatively charged particle was discovered by JJ. In the first the magnetic effect on cathode rays was studied while in the second the rays were deflected by an. Thomson performed three experiments with cathode ray tubes. First he used a magnet and electrometer to observe that the cathode rays were indeed electrically charged. Thomson began experimenting with cathode ray discharge tubes. Thomson conducted an experiment called the plum pudding model of the atom that involved passing an electric discharge through a region of gas. He did the study about electric discharge in high vacuum cathode. He zapped atoms with electricity and observed that. Thomson found that the cathode rays can be deflected by an electric field as shown below.

In 1897 Joseph John Thomson a British physicist proved that atoms are not the fundamental unit of matter. Previously atoms were known to be indivisible but in 1897 J.
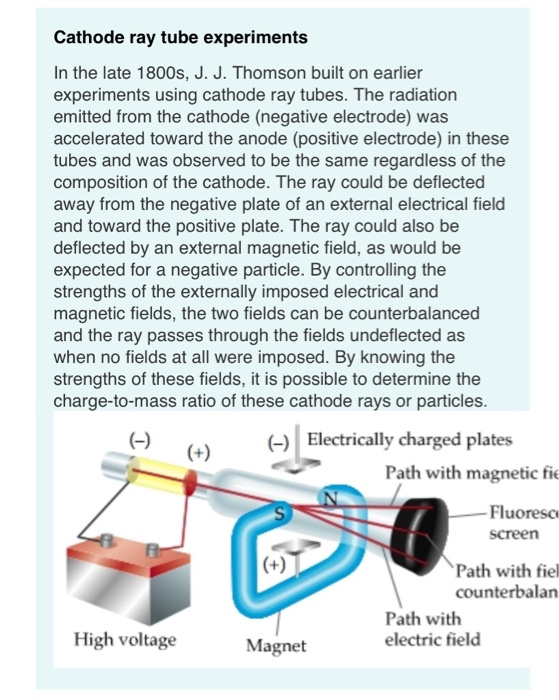
WHO PERFORMED THE CATHODE RAY EXPERIMENT SERIES
Thomson a highly respected theoretical physics professor at Cambridge University undertook a series of experiments. In 1897 JJ Thomson discovered the electron in his famous cathode ray tube experiment. JJ Thomsons cathode ray tube experiments. His entire works can be divided into three different experiments. Watch Ad Free Videos Completely FREE on Physicswallah Apphttpsbitly2SHIPW6Download the App from Google Play StoreDownload Lecture.ĭiscovery Of The Electron And Nucleus Article Khan Academy Atomic Structure Electrons Khan Academy Thomson a British physicist conducted the cathode ray experiment.


 0 kommentar(er)
0 kommentar(er)
