
When a monograph specifies that an article responds to the test for dry chlorides, mix the solid to be tested with an equal weight of manganese dioxide, moisten with sulfuric acid, and gently heat the mixture: chlorine, which is recognizable by the production of a blue color with moistened starch iodide paper, is evolved. Aqueous solution containing 20 g of ammonium chloride in 100 g.
manganese(II) bromide yes sodium sulfide zinc chloride yes potassium acetate ammonium nitrate YCs.

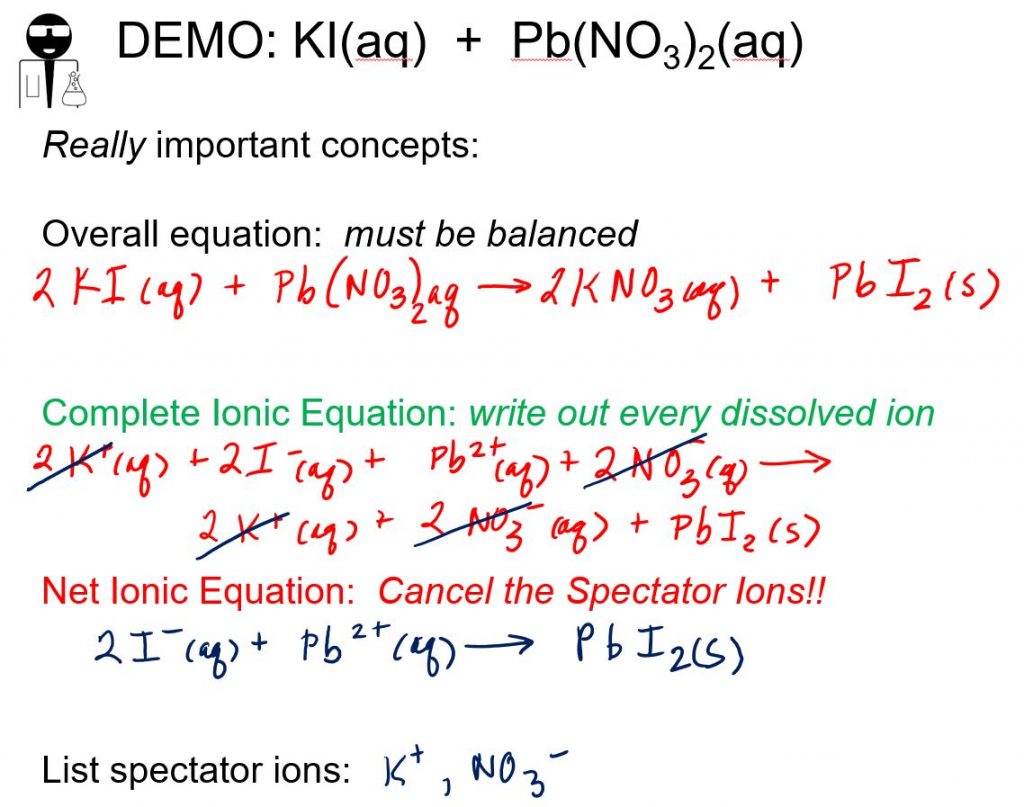
40-5135-05 Ammonium Acetate 7.5M DNA & RNA Precipitation Solution 50 mL. Then the next is lead nitrate with potassium iodide. What you have is a mixture of aqueous ions. lead nitrate aqueous solution at 0.01 g of lead. 40-5133-05 Potassium Acetate 3M pH 5.5 DNA & RNA Precipitation Solution. Add ammonia TS dropwise to this precipitate. In order for a double replacement to occur, one product must be a precipitate, insoluble gas, or water. Wash the precipitate with three 1-mL portions of nitric acid solution (1 in 100), and discard the washings. Centrifuge the mixture without delay, and decant the supernatant layer.

SCVFZCLFOSHCOH-UHFFFAOYSA-M Potassium acetate Chemical compound. When testing amine (including alkaloidal) hydrochlorides that do not respond to the above test, add one drop of diluted nitric acid and 0.5 mL of silver nitrate TS to a solution of the substance being examined containing, unless otherwise directed in the monograph, about 2 mg of chloride ion in 2 mL: a white, curdy precipitate is formed. calcium permanganate and ammonium peroxydisulfate are prepared from oxidizing agents. With silver nitrate TS, solutions of chlorides yield a white, curdy precipitate that is insoluble in nitric acid but is soluble in a slight excess of 6 N ammonium hydroxide. Potassium acetate C2H3O2K or CH3COOK or C2H3KO44 - structure, chemical names, physical and chemical properties, classification, patents.


 0 kommentar(er)
0 kommentar(er)
